Go deeper than the autism diagnosis
A diagnosis is only part of the story. Our genomic testing can reveal the cause behind your patient’s autism.
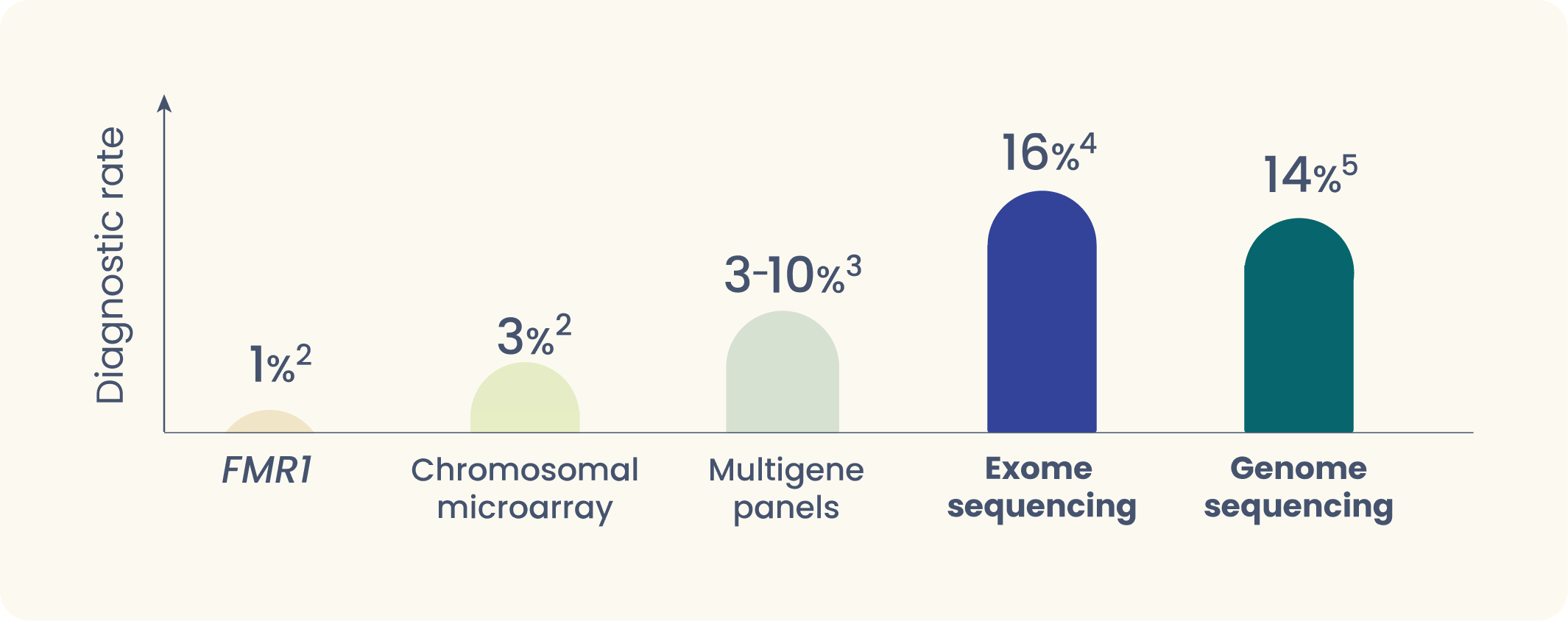
- Panel and CMA tests can miss many genes tied to autism
Providers often lean on tests such as chromosomal microarray (CMA), Fragile X (FMR1), and single- or multi-gene panel tests for answers. But those may only look at a fraction of the 1,000+ genes linked to autism. - Our genomic tests give you the full picture
By looking at ~20,000 disease-causing genes at once, our exome testing offers insights that others can overlook, delivered in concise reports that are easy for you to act on.


90% of patients went undiagnosed before getting an exome test
In a study of 18,000+ patients with autism, 90% received negative results from at least one prior genetic test.1 This highlights how broader testing can uncover answers that other tests miss.

Confidence in the insights. Support for what’s ahead.
Genetic answers can shape care, ease uncertainty, and empower families navigating autism.
Genetic testing for ASD can:4,8
- Provide genetic diagnoses.
- Identify areas of need and opportunities for support.
- Guide more personalized care, treatment, and medication decisions.
- Determine any co-occurring conditions.
- Connect families to gene-related support groups and resources.
- Call out recurrence risks that inform family planning.


A more complete picture of autism that’s backed by data—and trusted by providers and families alike.
Autism often goes hand in hand with other conditions. Exome testing makes it easier for you to connect the clinical dots.

of ASD patients have 1+ co-occurring conditions ASD is rarely an island. Nearly 3 out of 4 patients have at least one co-occurring condition, such as intellectual disability (ID) or epilepsy.⁶
diagnostic yield for patients managing ASD and other co-occurring features such as ID, congenital anomalies, or epilepsy.¹˒⁴˒⁷
Exome sequencing stands out as the most accurate, reliable way to diagnose ASD and understand its genetic causes.4,5
Genome and exome sequencing are recommended as a first-line test by leading medical societies
- The National Society of Genetic Counselors (NSGC) recommends genome or exome as a first-line test for all individuals with unexplained epilepsy and the American Epilepsy Society (AES 10 endorsed this guideline).
- The American College of Medical Genetics and Genomics (ACMG) recommends genome or exome as a first-line test for developmental delay, intellectual disability, and congenital anomalies9.
- The International Precision Child Health Partnership (IPCHiP) recommends genome or exome as a first-line test for NICU patients with unexplained hypotonia5.
- The American Academy of Pediatrics (AAP) recommends ordering exome and genome as first-line tests for children with global developmental delays and intellectual disabilities11.


Find answers with GeneDx
We can help you take a guideline-driven approach.
Helpful resources
Clinical practice guidelines
The ACMG9, NSGC10, and AAP11 recommend or endorse exome and genome testing as first-line tests for multiple indications for conditions that commonly co-occur with autism.9,10,11
Removing cost barriers through genetic testing programs
Through our Autism Partnership Program, SHANK3 Genetic Testing, eligible patients who meet eight clinical criteria can receive guideline-backed exome testing with financial support.
How to order
Ordering genomic testing through GeneDx is straightforward, and our team is with you every step. Visit our ordering portal to choose the right test, submit required information, and send in your sample.




.svg)