A targeted, high-yield approach
Exome sequencing targets the protein-coding regions of the genome, where the majority of pathogenic variants are found.2 This makes exome testing an efficient option for patients with unexplained anomalies.
- A higher diagnostic rate that is 2x greater than a chromosomal microarray.4,6
- More likely to deliver answers than a panel, with 23% of exome diagnoses coming from findings panels would miss.7


Recommended as a first-line test
- The American College of Medical Genetics and Genomics recommends exome or genome as a first-tier test8 for developmental delay, intellectual disability, and congenital anomalies.
- The National Society of Genetic Counselors recommends exome or genome sequencing for all individuals with unexplained epilepsy.9 This guideline is endorsed by the American Epilepsy Society.
- The American Academy of Pediatrics (AAP) recommends ordering exome and genome as first-line tests for children with global developmental delays and/or intellectual disabilities.10


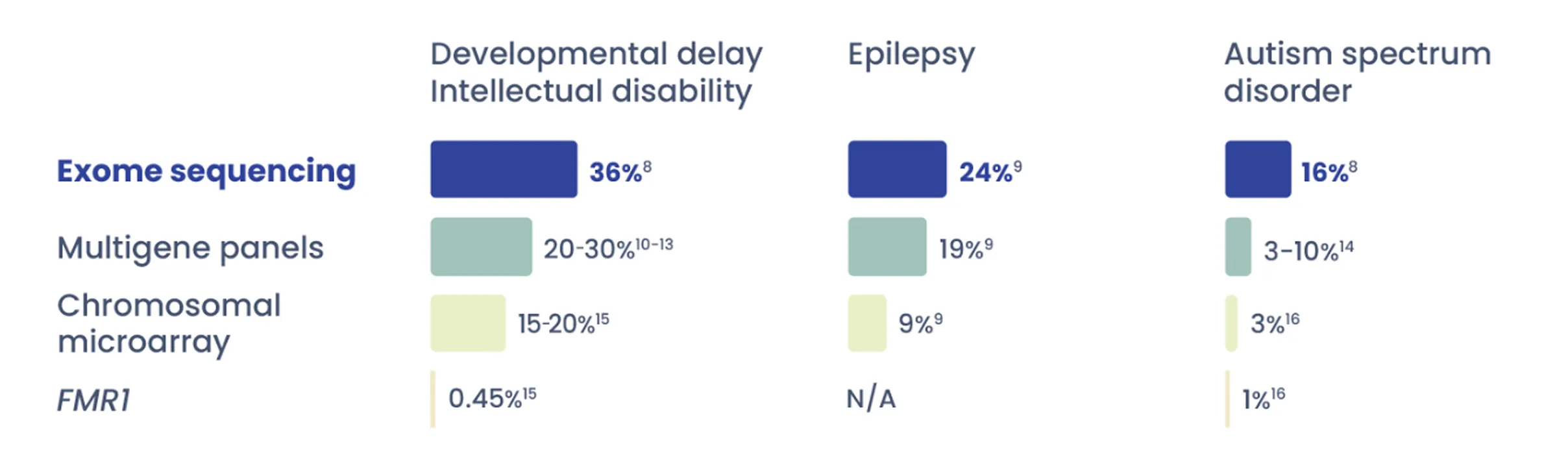
Diagnostic rate of exome sequencing vs. other technologies
Comprehensive clarity. One test.
Exome’s targeted approach increases the likelihood of identifying actionable findings in patients with a clear or suspected genetic phenotype.
Sequence variants
SNVs, indels, splice-site variants, and small intronic variants near exons
Copy-number variants
Exon-level deletions and duplications detected through exome-based CNV analysis
Mitochondrial variants (with ExomeDx + mito)
mtDNA SNVs and deletions
Inheritance insights
Trio or duo testing can improve interpretation confidence and reduce uncertainty
A complete suite of exome sequencing solutions
We offer multiple exome options that adapt to urgency and clinical need.
Choose a test
Use the test catalog to search by condition, gene, or indication. Each listing includes recommended use cases, methodology, and sample requirements—helping you select the right test for each patient.
Submit a sample
Request a sample collection kit, collect the sample, and ship it to our lab using the provided materials. Detailed instructions are included with every kit.
Receive results
Results are delivered securely through our online ordering portal, typically within 2–4 weeks.3 Each report includes clear findings, interpretation, and clinical guidance to support next steps in care.
Helpful resources
Billing and insurance
Learn more about how billing works at GeneDx and the variety of support tools we offer.
Support
Our team of clinical and genetic experts are here throughout the process—from education and guidance to results interpretation and next steps.

%201.webp)